Sterile Injectable Drugs Market to Expand Significantly, Projected to Achieve USD 1470.3 Billion by 2033
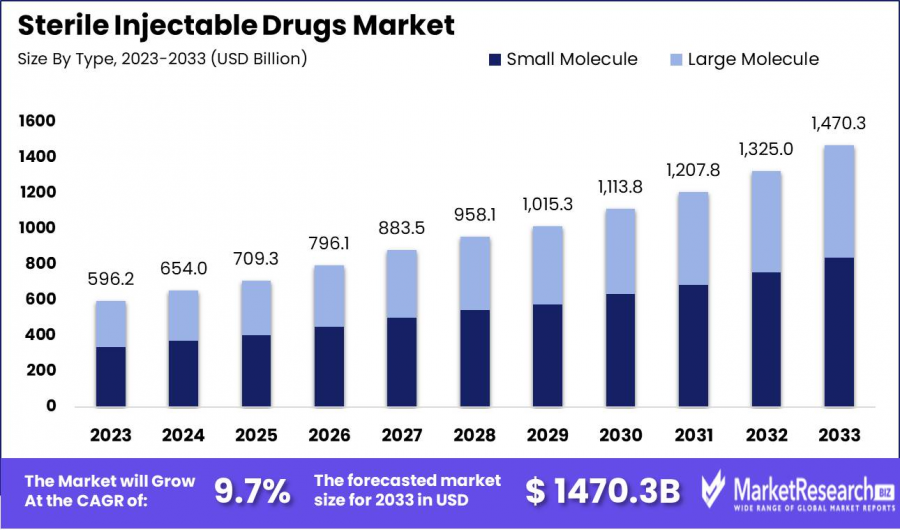
The Sterile Injectable Drugs Market was valued at USD 596.2 billion in 2023. It is expected to reach USD 1470.3 billion by 2033, with a CAGR of 9.7%
NEW YORK, NY, UNITED STATES, February 18, 2025 /EINPresswire.com/ -- Overview
The Sterile Injectable Drugs Market was valued at USD 596.2 billion in 2023. It is expected to reach USD 1470.3 billion by 2033, with a CAGR of 9.7% during the forecast period from 2024 to 2033.
The global sterile injectable drugs market is experiencing significant growth, driven by rising demand for biologics, vaccines, and emergency care treatments. Sterile injectable drugs are medications administered directly into the bloodstream, ensuring rapid absorption and immediate therapeutic effects. These drugs are widely used in hospitals, clinics, and ambulatory care settings for treating chronic diseases, infections, and pain management.
With an increasing prevalence of cancer, cardiovascular diseases, and autoimmune disorders, the need for injectable biologics and biosimilars is expanding. Additionally, technological advancements in manufacturing and stringent regulatory standards ensure the highest safety and efficacy of these drugs.
Key pharmaceutical companies are focusing on expanding their production capacities and investing in advanced formulations to meet the rising demand. The COVID-19 pandemic further accelerated the adoption of sterile injectables, especially in vaccine administration and intensive care treatments.
As healthcare providers prioritize patient safety and efficacy, the sterile injectable drugs market is expected to witness sustained growth. With ongoing research and development, new drug formulations and innovative delivery systems are expected to enhance treatment outcomes, making sterile injectables a vital component of modern medicine.
Click here to get a Sample report copy @ https://marketresearch.biz/report/sterile-injectable-drugs-market/request-sample/
Key Takeaways
•Market Growth: The Sterile Injectable Drugs Market was valued at USD 596.2 billion in 2023 and is projected to reach USD 1,470.3 billion by 2033, expanding at a CAGR of 9.7% between 2024 and 2033.
•By Type: Small Molecule injectables held the dominant market share in 2023.
•By Therapeutic Application: Cardiovascular Diseases emerged as the leading segment in therapeutic applications.
•By Distribution Channel: Hospital Pharmacies remained the primary distribution channel for sterile injectable drugs.
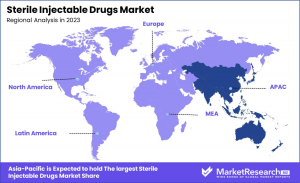
•Regional Dominance: Asia Pacific accounted for the largest market share, holding 35% of the total market.
•Growth Opportunity: The market's expansion is driven by biotechnology advancements and the development of healthcare infrastructure, which enhance drug availability and accessibility.
Segmentation Analysis
•By Type Analysis: In 2023, Small Molecule sterile injectables dominated the market due to their broad therapeutic applications in cardiovascular, oncological, and infectious diseases. Their affordability, simpler manufacturing, and faster regulatory approvals contributed to their strong market presence. Meanwhile, the Large Molecule segment is expanding rapidly, driven by biotech advancements and increasing demand for biologics in personalized medicine. Despite their higher costs and complex production, their high specificity and efficacy position them for significant future market growth.
•By Therapeutic Application Analysis: In 2023, Cardiovascular Diseases led the sterile injectable drugs market, driven by the high prevalence and chronic nature of these conditions. Cancer treatments also held a significant share due to advancements in targeted therapies and increasing oncology cases. Diabetes management remained critical, while infectious disorders gained traction due to global efforts against emerging pathogens. Neurological and musculoskeletal disorders also expanded, reflecting growing demand for innovative treatments in aging populations and chronic disease management.
•By Distribution Channel Analysis: In 2023, Hospital Pharmacies dominated sterile injectable drug distribution due to their controlled environment, advanced storage systems, and professional administration requirements. Retail pharmacies played a supportive role in chronic disease management, while online pharmacies saw growth driven by telemedicine and e-commerce advancements. However, challenges like cold chain logistics and regulatory hurdles currently limit their expansion. As digital health adoption increases, online distribution channels are expected to gain a stronger foothold in the market.
Market Segments
By Type
•Small Molecule
•Large Molecule
By Therapeutic Application
•Cancer
•Diabetes
•Cardiovascular Diseases
•Infectious Disorders
•Central Nervous Systems
•Musculoskeletal
•Anti-Viral
•Others
By Distribution Channel
•Hospital Pharmacies
•Retail Pharmacies
•Online Pharmacies
To Purchase this Premium Report @ https://marketresearch.biz/purchase-report/?report_id=47392
Market Dynamics
-Driver: The sterile injectable drugs market is primarily driven by the increasing prevalence of chronic diseases such as cancer, diabetes, and cardiovascular disorders. These conditions often require rapid and effective therapeutic interventions, for which sterile injectables are preferred due to their immediate bioavailability.
Additionally, advancements in biotechnology have led to the development of complex biologics and biosimilars, many of which are administered via injection. The necessity for precise dosing and targeted delivery in treatments further propels the demand for sterile injectable formulations. Moreover, the global aging population contributes to a higher incidence of chronic ailments, thereby escalating the need for injectable therapeutics.
-Trend: A significant trend in the sterile injectable drugs market is the shift towards the outsourcing of manufacturing processes to contract development and manufacturing organizations (CDMOs). This approach allows pharmaceutical companies to leverage specialized expertise and advanced technologies, ensuring compliance with stringent regulatory standards.
The COVID-19 pandemic has also underscored the importance of flexible and scalable production capacities, prompting firms to adopt modular manufacturing solutions. Furthermore, there is a growing emphasis on the development of biosimilars and generic injectables to make treatments more accessible and affordable. Technological innovations, such as pre-filled syringes and auto-injectors, are enhancing patient convenience and adherence to therapeutic regimens.
-Restraint: One of the primary restraints in the sterile injectable drugs market is the complexity and high cost associated with manufacturing processes. Maintaining aseptic conditions requires significant investment in specialized facilities and equipment. Additionally, stringent regulatory requirements necessitate comprehensive validation and quality assurance protocols, which can prolong product development timelines and escalate costs.
The market has also faced challenges related to drug shortages, often stemming from manufacturing disruptions and quality control issues. For instance, the U.S. Food and Drug Administration (FDA) has reported instances where manufacturing plants failed to comply with sterile production standards, leading to supply constraints.
-Opportunity: The sterile injectable drugs market presents substantial opportunities, particularly in the realm of biologics and personalized medicine. The increasing incidence of complex diseases has spurred research into targeted therapies, many of which are administered via sterile injection. Emerging markets are also witnessing an expansion of healthcare infrastructure, enhancing access to advanced treatments and driving demand for injectable drugs.
Collaborations between pharmaceutical companies and CDMOs can facilitate the efficient scaling of production capacities to meet global needs. Moreover, technological advancements in manufacturing processes, such as automation and continuous production techniques, offer the potential to reduce costs and improve product quality, thereby addressing some of the existing market restraints.
Market Key Players
•GlaxoSmithKline PLC
•Baxter International Inc.
•AstraZeneca
•Johnson & Johnson Services, Inc.
•Sanofi
•Merck & Co., Inc.
•Gilead Sciences Inc.
•Pfizer Inc.
•Novartis AG
•Nova Nordisk A/S
•Amgen, Inc.
Regional Analysis
The Sterile Injectable Drugs Market demonstrates diverse regional trends, influenced by healthcare infrastructure, regulatory frameworks, and market demand. North America holds a significant share, supported by a well-established healthcare system, high R&D investments, and a strong presence of major pharmaceutical companies. The U.S. leads in this region, with a vast portfolio of FDA-approved sterile injectables.
Europe follows, with stringent regulatory standards and a growing biosimilars market, particularly in Germany, France, and the U.K., where healthcare spending remains high. Asia Pacific, however, dominates with a 35% market share, driven by expanding healthcare infrastructure, a rising burden of chronic diseases, and increased pharmaceutical investments, especially in China and India.
In contrast, the Middle East & Africa is experiencing gradual growth, supported by government initiatives and improved healthcare access, though market penetration remains relatively lower. Latin America is witnessing steady progress, with Brazil and Mexico emerging as key contributors due to large patient populations and healthcare reforms.
Overall, Asia Pacific is poised to maintain its leadership, supported by favorable demographic trends, increasing demand for biologics, and strong investments in sterile drug manufacturing, positioning the region as a critical growth driver in the global market.
Emerging Trends in Sterile Injectable Drugs
••Manufacturing Challenges and Drug Shortages: There is a significant shortage of critical drugs, particularly sterile injectables. As of May 2023, 16 oncology drugs, including fludarabine and methotrexate injections, were in short supply. Factors contributing to these shortages include manufacturing issues, regulatory challenges, increased demand, and supply chain disruptions.
••Quality Control and Safety Concerns: Recurring problems in drug manufacturing, such as microbial contamination and poor quality of ingredients, have been identified. Instances include contamination of ophthalmic products with multi-drug resistant bacteria and the presence of harmful substances like diethylene glycol in drug products.
••Regulatory and Compliance Efforts: The FDA emphasizes the importance of adhering to Current Good Manufacturing Practices (CGMP) to ensure drug safety and effectiveness. This includes proper facility design, equipment maintenance, and rigorous quality control measures.
Use Cases of Sterile Injectable Drugs
••Oncology Treatments: Many cancer therapies rely on sterile injectables. For instance, methotrexate injection is used to treat certain types of cancer. However, as of May 2023, methotrexate injection was among the 16 oncology drugs reported to be in shortage.
••Anesthesia Administration: Sterile injectable anesthetics are crucial during surgeries. Drugs like cisatracurium besylate are used to facilitate muscle relaxation. During the COVID-19 pandemic, the FDA provided guidance on extending the in-use times of such drugs to manage supply challenges.
••Emergency Medicine: In critical care, injectable drugs like epinephrine are vital for treating severe allergic reactions and cardiac arrest. Ensuring the sterility and availability of these injectables is paramount, as contamination can lead to serious health risks.
Explore More Healthcare Reports
Pharmacokinetics Services Market-https://marketresearch.biz/report/pharmacokinetics-services-market/
Pharmaceutical Analytical Testing Outsourcing Market-https://marketresearch.biz/report/pharmaceutical-analytical-testing-outsourcing-market/
Pet Waste Bags Market-https://marketresearch.biz/report/pet-waste-bags-market/
Pseudomonas Aeruginosa Infection Treatment Market-https://marketresearch.biz/report/pseudomonas-aeruginosa-infection-treatment-market/
Peptide and Anticoagulant Drugs Market-https://marketresearch.biz/report/peptide-and-anticoagulant-drugs-market/
Patient Recliner Market-https://marketresearch.biz/report/patient-recliner-market/
Patient Handling Equipment Market-https://marketresearch.biz/report/patient-handling-equipment-market/
PAP and Paracetamol Market-https://marketresearch.biz/report/pap-and-paracetamol-market/
Oxygen Conserving Devices Market-https://marketresearch.biz/report/oxygen-conserving-devices-market/
Onychomycosis Treatment Market-https://marketresearch.biz/report/onychomycosis-treatment-market/
Pigmented Lesion Treatment Market-https://marketresearch.biz/report/pigmented-lesion-treatment-market/
Lawrence John
Prudour
91308 55334
Lawrence@prudour.com
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release